Organic Chemistry Substitution Reaction
Predict the major products for the reaction and show the full mechanism

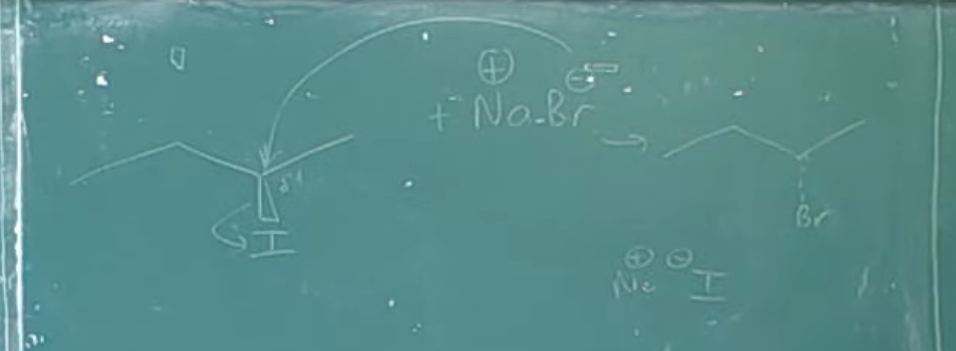
An SN2 reaction, which stands for substitution, nucleophilic, bimolecular, is a fundamental mechanism in organic chemistry. The term "bimolecular" indicates that the rate-determining step involves two reactant molecules: the nucleophile and the alkyl halide. The defining characteristic of the SN2 mechanism is its single-step process without any intermediates. During this reaction, the nucleophile attacks the alkyl halide (or tosylate) from the side opposite the leaving group. As the nucleophile forms a bond with the carbon atom, the leaving group simultaneously departs from the other side. This concerted mechanism results in the inversion of the stereochemical configuration of the substrate.

The mechanism of the SN2 reaction. The reaction takes place in a single step when the incoming nucleophile approaches from a direction 180° away from the leaving halide ion, thereby inverting the stereochemistry at carbon.
1. The nucleophile -OH uses its lone-pair electrons to attack the alkyl halide carbon 180 degrees away from the departing halogen. This leads to a transition state with a partially formed C-OH bond and a partially broken C-Br bond.
2. The stereochemistry at carbon is inverted as the C-OH bond forms fully and the bromide ion departs with the electron pair from the former C-Br bond.
Related Problems
Predict the major products for the reaction and show the full mechanism

Predict the major products for the reaction and show the full mechanism

Predict the major products for the reaction and show the full mechanism
