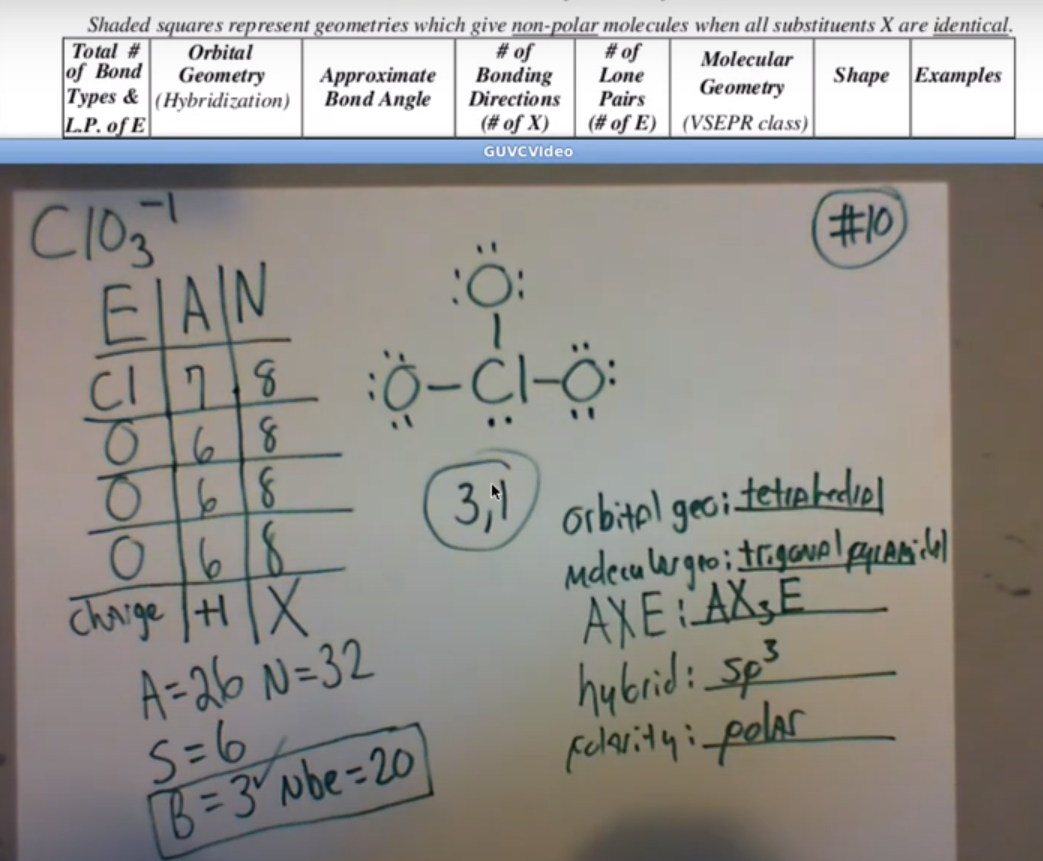
ClO3- Lewis Structure
Provide the lewis dot structure, molecular geometry, orbital geometry, polarity, and the hybridization ClO
A simple way of indicating the covalent bonds in molecules is to use what are called Lewis structures, or electron-dot structures, in which the valence-shell electrons of an atom are represented as dots. Thus, hydrogen has one dot representing its 1s electron, carbon has four dots (2s2 2p2), oxygen has six dots (2s2 2p4), and so on. A stable molecule results whenever a noble-gas configuration of eight dots (an octet) is achieved for all main-group atoms or two dots for hydrogen. Even simpler than Lewis structures is the use of Kekulé structures, or line-bond structures, in which the two-electron covalent bonds are indicated as lines drawn between atoms.
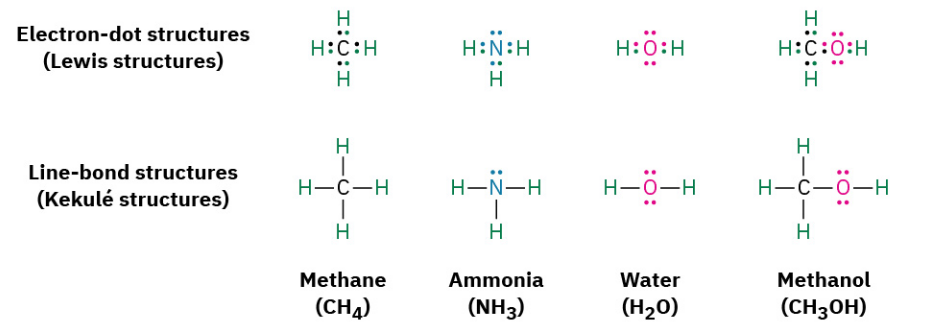
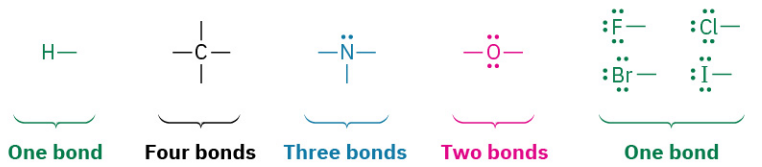
The number of covalent bonds an atom forms depends on how many additional valence electrons it needs to reach a noble-gas configuration. Hydrogen has one valence electron (1s) and needs only one more to reach the helium configuration (1s2), so it forms one bond. Carbon has four valence electrons (2s2 2p2) and needs four more to reach the neon configuration (2s2 2p6), so it forms four bonds. Nitrogen has five valence electrons (2s2 2p3), needs three more, and forms three bonds; oxygen has six valence electrons (2s2 2p4), needs two more, and forms two bonds; and the halogens have seven valence electrons, need one more, and form one bond.
Related Problems
Provide the lewis dot structure, molecular geometry, orbital geometry, polarity, and the hybridization of CO
Provide the lewis dot structure, molecular geometry, orbital geometry, polarity, and the hybridization of NH
Provide the lewis dot structure, molecular geometry, orbital geometry, polarity, and the hybridization of IF
Provide the lewis dot structure, molecular geometry, orbital geometry, polarity, and the hybridization of SO